
how to identify an atom
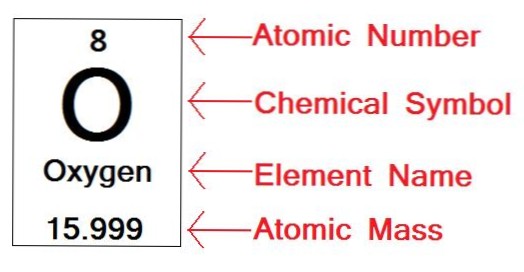
There are two properties that can be used to identify an element: the atomic number or the number of protons in an atom. The number of neutrons and number of electrons are frequently equal to the number of protons, but can vary depending on the atom in question.
- How do you find the identity of an atom?
- How do scientists identify an atom?
- What causes an atom to be neutral?
- What is the smallest thing in the world?
- Do atoms die?
- Why can't scientists see inside an atom?
- Is an atom electrically neutral?
- How do you tell if an atom is an ion?
- How do you know if an atom is neutral?
How do you find the identity of an atom?
The number of protons in one atom of an element determines the atom's identity, and the number of electrons determines its electrical charge. The atomic number tells you the number of protons in one atom of an element. It also tells you the number of electrons in a neutral atom of that element.
How do scientists identify an atom?
It might seem as if there's a simple way to prove atoms exist: put them under the microscope. ... Atoms are so much smaller than the wavelength of visible light that the two don't really interact. To put it another way, atoms are invisible to light itself.
What causes an atom to be neutral?
When an atom has an equal number of electrons and protons, it has an equal number of negative electric charges (the electrons) and positive electric charges (the protons). The total electric charge of the atom is therefore zero and the atom is said to be neutral.
What is the smallest thing in the world?
Protons and neutrons can be further broken down: they're both made up of things called “quarks.” As far as we can tell, quarks can't be broken down into smaller components, making them the smallest things we know of.
Do atoms die?
Since an atom has a finite number of protons and neutrons, it will generally emit particles until it gets to a point where its half-life is so long, it is effectively stable. ... It undergoes something known as “alpha decay,” and it's half-life is over a billion times longer than the current estimated age of the universe.
Why can't scientists see inside an atom?
Almost all of an atom's mass comes from the protons and neutrons in the nucleus. However, because electrons orbit around the nucleus, most of an atom is empty space! ... You can't see atoms with the naked eye, because they're simply too small. Using electron microscopes, scientists have been able to study atoms.
Is an atom electrically neutral?
So an atom as a whole is electrically neutral. When one or more electrons is stripped away from an atom, it becomes positively charged. Some atoms can attract additional electrons so they become negatively charged. Atoms which are not electrically neutral are called ions.
How do you tell if an atom is an ion?
When an atom is attracted to another atom because it has an unequal number of electrons and protons, the atom is called an ION. If the atom has more electrons than protons, it is a negative ion, or ANION. If it has more protons than electrons,it is a positive ion.
How do you know if an atom is neutral?
A proton and an electron have an equal amount but an opposite type of charge. Thus, if an atom contains equal numbers of protons and electrons, the atom is described as being electrically neutral.



Yet No Comments